Introduction
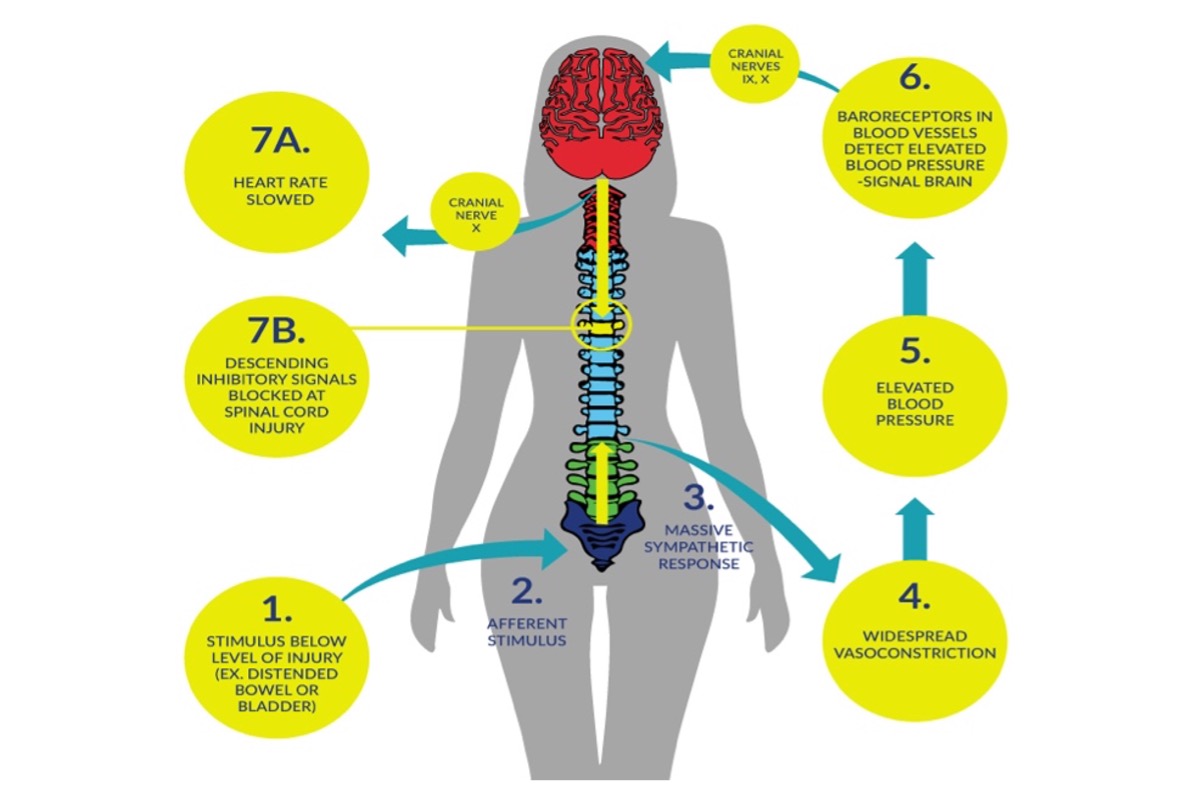
Autonomic dysreflexia (AD) is a clinical emergency that can occur in individuals with spinal cord injury (SCI), commonly in those with an injury at level T6 or above (Mathias & Frankel 1988; Mathias & Frankel 2002; Teasell et al. 2000). An episode of AD is usually characterized by an acute elevation of arterial blood pressure (BP) and bradycardia (slow heart rate), which may be replaced by tachycardia (rapid heart rate) on occasion. Objectively, an increase in systolic BP greater than 20–30mmHg is considered a dysreflexic episode (Teasell et al. 2000). Individuals with cervical and high thoracic SCI have resting arterial BPs that are approximately 15 to 20 mmHg lower than individuals without SCI (Mathias & Bannister 2002; Claydon & Krassioukov, 2006). As such, acute elevation of BP to normal or slightly elevated ranges could indicate AD in this population. Intensity of AD can vary from asymptomatic (Linsenmeyer et al. 1996), to mild discomfort and headache, to a life threatening emergency where systolic blood pressure can reach 300mmHg and symptoms can be severe (Mathias & Bannister 2002). Untreated episodes of AD may have serious consequences, including intracranial hemorrhage, cardiac complications, retinal detachments, seizures, and death (Eltorai et al. 1992; Pine et al. 1991; Vallès et al. 2005; Yarkony et al. 1986). During an episode of AD, a significant increase in visceral sympathetic activity with coronary artery constriction can result in myocardial ischemia, even in the absence of coronary artery disease (Ho & Krassioukov 2010).
It has been observed that higher level of SCIs equate to greater degrees of cardiovascular dysfunctions (Curt et al. 1997; Krassioukov et al. 2003). Another factor affecting the severity of AD is the degree of completeness of SCI as AD is three times more prevalent in people with complete tetraplegia (91% of individuals presenting with signs of AD) compared to those with incomplete tetraplegia (27% of individuals presenting with signs) (Curt et al. 1997). However, it is important to note that although autonomic dysreflexia occurs more often in the chronic stage of spinal cord injury at or above the 6th thoracic segment, there is clinical evidence of early episodes of autonomic dysreflexia within the first days and weeks after the injury (Krassioukov et al. 2003; Silver, 2000).