Primary Care and Orthostatic Hypotension
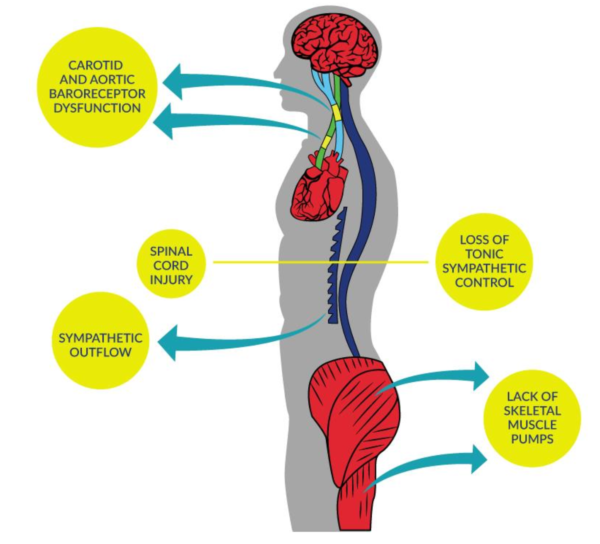
Blood pressure (BP) instability is common in people with SCI and up to 4x more likely in people with higher level injuries (i.e., tetraplegia or cervical).
Normally, the nervous system automatically constricts or dilates the blood vessels to balance blood pressure, but after a SCI this ability may be compromised, particularly for people with injuries at or above T6.
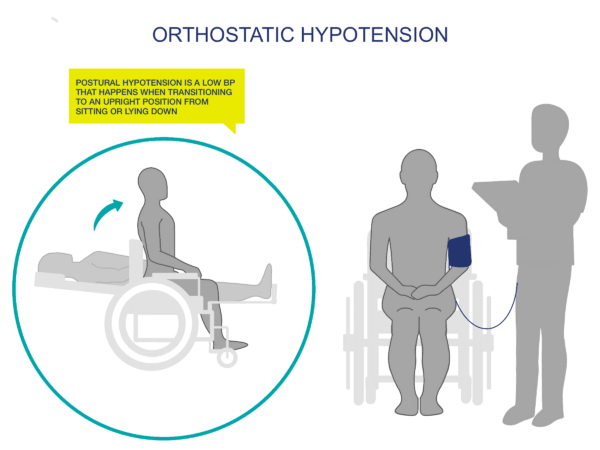
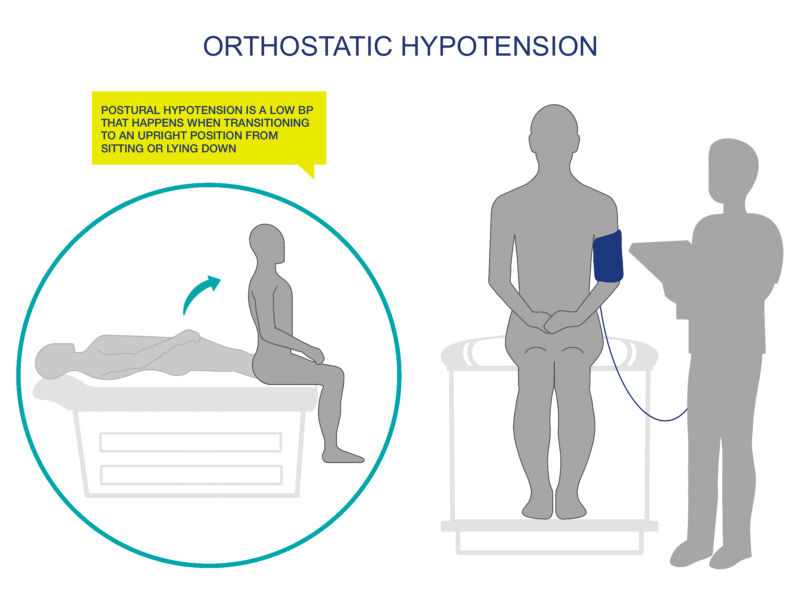
Orthostatic hypotension (OH) is defined as a decrease in systolic blood pressure of at least 20mmHg, or a reduction in diastolic blood pressure of at least 10mmHg, upon the change in body position from a supine (lying) to an upright position.
Symptoms may or may not occur during an episode of OH, but common signs and symptoms include:
• Temporary loss of consciousness
• Fainting
• Dizziness
• Light-headedness
• Fatigue
• Blurry vision
• Muscle weakness
Multiple large cohort studies have shown that delayed BP recovery and classic OH are associated with future risk of falls, fractures, fainting, stroke, CVD, and even earlier death (10-year mortality rate of 50-64%). Something as simple as sitting up or eating may trigger blood pressure decreases and cause symptoms of OH, discouraging people with SCI from participating in rehabilitation, exercise, or completing activities of daily living.
If your patient has hypotension with symptoms, the best thing you can do is prioritize symptom improvements instead of targeting a BP value. Approaches should be tailored to the individual due to complexities in SCI.
First-line treatment recommendations are:
- Medication Review – all medications should be reviewed for unwanted cardiovascular/hypertensive/hypotensive effects and then replaced or discontinued as appropriate (alpha-antagonists and/or beta-blockers can exacerbate OH by diminishing compensatory cardiovascular responses).
- Education and Counselling – early recognition/warning signs of symptoms and avoidance of triggering events (e.g., straining, too quickly rising from supine or sitting positions, heavy meals with high carbohydrate or alcohol, hot environments like showers or saunas). Encouraging adaptations and educating patients and family members are helpful.
- Physical Counter Maneuvers – may be difficult to implement in neurologically driven OH, but rapid reclining can often alleviate OH – should be tested and measured by doctors/under supervision. Ask patient to avoid sitting up too quickly after sleeping can help them avoid the dizziness and fainting effects of hypotension.
- Dietary Measures – Rapid Ingestion of Water – 500 ml ingested in 2-3 minutes usually raises BP within 5-10 minutes and the effect can last for 30-45 minutes. Eating smaller and more frequent meals – OH often occurs in people with SCI after eating when blood rushes to the digestive system. Increasing daily salt intake and monitoring weight – people often adopt heart-healthy diets with lower sodium content, but in the instance of hypotension, it may be counterproductive. Salt supplementation promotes plasma volume expansion and may have a positive effect on OH.
- Regular (moderate) physical activity usually has a positive effect on OH through better blood flow and avoidance of physical deconditioning. Compression socks, which put light pressure on legs and feet, can help push blood upward and raise patient’s blood pressure.
If none of the above are successful, pharmacological treatment options may then be considered. Midodrine (a drug that constricts the blood vessels to bring up blood pressure) has some evidence supporting its use in people with SCI as it can elevate blood pressure and improve exercise performance, though its use should be monitored carefully as side effects like urinary bladder dysreflexia have been reported.
People with OH will most often have an injury level of T6 or above, but it is also more likely to occur in your patients that are older (>50).
OH also frequently occurs without symptoms, so measuring your patient’s usual (“normal”) blood pressure while standing, sitting, and lying down (supine) will be helpful. Document these baseline values and update as necessary (at least annually).
Baseline BP will generally be lower for people with SCI (100/60 mmHg +/- 20). It is important to establish your patient’s baseline standing, sitting, and lying (supine) BP to properly monitor fluctuations and to implement preventative measures.
Some people with SCI (i.e., those with incomplete SCI) will be able to do a modified sit to stand test for you to test BP changes. Some will have to do a lying to sitting test if they can transfer to an examination table, or if they have a wheelchair that reclines. Lying to sitting BP tests should be able to give you the information that you need re: BP changes.
If treatment options such as education, diet management, compression socks, exercise, and/or medication review are insufficient to resolve your patient’s OH, you may need to refer them to a specialist (e.g., a physiatrist or a cardiologist) for further examination.
It is also more likely that referral is advisable if your patient is older, has a longer duration of injury, has multiple health problems, and/or is significantly physically deconditioned (i.e., in poor physical health). You may also need to refer your patient with SCI and OH to specialist care because the number of episodes and severity of symptoms is significantly interfering with your patient’s daily activities (e.g., work, school, self-care).
You may need to send your patient to the Emergency Room (ER) if they have chest pain, if they are passing out, hurt themselves falling, or exhibit any symptoms of shock like feeling cold, increased heart rate, or having a blue tint to their lips or under their fingernails.
Access to specialists (e.g., physiatry) may be limited, particularly if you do not live in or near a major city.
If you or your patients with SCI are not already connected, please try to gain access to a urologist or physiatrist near you. Refer to the list of SCI centers worldwide below:
- Australia (Queensland Spinal Cord Injuries Service): https://www.health.qld.gov.au/qscis
- Canada: https://praxisinstitute.org/research-care/key-initiatives/national-sci-registry/registry-sites
- Europe (enrolled in European Multicenter Spinal Cord Injury Study): https://www.emsci.org/index.php/members
- UK: https://www.brainandspine.org.uk/wp-content/uploads/2018/03/BSF_List-of-Neurocentres-in-the-UK.pdf
- USA: https://www.spinalcord.com/spinal-cord-injury-hospitals-rehabilitation-directory
(Please email SCIRE Professional if we do not have a list for your country)
Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular Consequences of Loss of Supraspinal Control of the Sympathetic Nervous System Following Spinal Cord Injuries. Arch Phys Med Rehabil 2000;81:506-516
Cleveland Clinic. Low Blood Pressure (Hypotension) Accessed on March 13, 2023.
American Association of Physical Medicine & Rehabilitation. Orthostatic Hypotension in SCI. Accessed on September 1, 2023. https://now.aapmr.org/orthostatic-hypotension-in-sci/
Bhatt SP. Therapeutic Aproach to the Hypotensive Patient. In: Cydulka RK, Fitch MT, Joing SA, Wang VJ, Cline DM, Ma O, eds. Tintinalli’s Emergency Medicine Manual. 8th ed. McGraw Hill; 2017.
Sharma S, Jashmi MF, Bhattacharya PT. Hypotension. 2021 Sep. 14. In: StatPearls (Internet). Treasure Island, FL: StatPearls Publishing; 2021. Accessed March 13, 2023.
Cautela J, Tartiere JM, Cohen-Solal A, Bellemain-Appaix A, Theron A, Tibi T, Januzzi JL, Roubille F, Girerd N. Management of low blood pressure in ambulatory heart failure with reduced ejection fraction patients. Eur J heart Fail 2020; 22: 1357-1365.
Katzelnick CG, Weir JP, Jones A, Galea M, Dyson-Hudson TA, Kirshblum SC, Wecht JM. Blood pressure instability in persons with SCI: Evidence from a 30-day home monitoring observation. American Journal of Hypertension 2019; 32(10): 938-944.
Wieling W, Kaufmann H, Claydon VE, van Wijnen VK, Harms MPM, Juraschek SP, Thijs RD. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol 2022; 21: 735-46.
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a National Population Health Survey. Neurology 2013; 81:723–728.
Dance DL, Chopra A, Campbell K, Ditor DS, Hassouna M, Craven BC. Exploring daily blood pressure fluctuations and cardiovascular risk
among individuals with motor complete spinal cord injury: a pilot study. J Spinal Cord Med 2017; 40:405–414.
Claydon VE, Krassioukov AV. Clinical correlates of frequency analyses of cardiovascular control after spinal cord injury. Am J Physiol Heart Circ Physiol 2008; 294:H668–H678.
West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 2012; 50:484–492.
Sahota IS, Ravensbergen HR, McGrath MS, Claydon VE. Cerebrovascular responses to orthostatic stress after spinal cord injury.
J Neurotrauma 2012; 29:2446–2456.
Huang J. Low diastolic blood pressure as a risk for all-cause mortality in VA patients. Int J Hypertens 2013: 1–5.
Krassioukov A, Biering-Sørensen F, Donovan W, Kennelly M, Kirshblum S, Krogh K, Alexander MS, Vogel L, Wecht J; Autonomic
Standards Committee of the American Spinal Injury Association/ International Spinal Cord Society. International standards to document
remaining autonomic function after spinal cord injury. J Spinal Cord Med 2012; 35:201–210.
Zhu C, Galea M, Livote E, Signor D, Wecht JM. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J Spinal Cord Med 2013; 36:463–475.
Wecht JM, Zhu C, Weir JP, Yen C, Renzi C, Galea M. A prospective report on the prevalence of heart rate and blood pressure abnormalities
in veterans with spinal cord injuries. J Spinal Cord Med 2013; 36:454–462.
Rosado-Rivera D, Radulovic M, Handrakis JP, Cirnigliaro CM, Jensen AM, Kirshblum S, Bauman WA, Wecht JM. Comparison of
24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med 2011; 34:395–403.
Krassioukov A, Eng JJ, Warburton DE, Teasell R. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil. 2009;90:876-885.
Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma. 2006;23:1713-1725.
The Consensus Committee of the American Autonomic Society and the American Academy of Neurology 1. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
Palma JA, Kaufmann H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov Disord Clin Pract 2017;
4: 298–308.
Fedorowski A, Ricci F, Hamrefors V, et al. Orthostatic hypotension: management of a complex, but common, medical problem.
Circ Arrhythm Electrophysiol 2022; 15: e010573.
van Dijk JG, van Rossum IA, Thijs RD. Timing of circulatory and neurological events in syncope. Front Cardiovasc Med 2020;
7: 36.
Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology 2015;
85: 1362–67.
For Clinical Practice Guideline recommendations based on your patient’s level of injury and specific complaint, click here for the CAN-SCIP Coach App.
For more information see our Orthostatic Hypotension Module.
Disclaimer
All content and information on this website is for informational and educational purposes only, does not constitute medical advice, and does not establish any kind of patient-client relationship by your use of this website. The information here has been curated by experts in spinal cord injury treatment but it is not a substitute for actual medical consultation. Always see a medical professional directly for specific treatment for your particular needs including any professional, health-related, legal, medical, or financial decisions.