Spinal Cord Stimulation Combined With Locomotor Training (LT)
The discovery of central pattern generators in the spinal cord has led to testing different methods of electrical stimulation to restore or force patterned locomotion (Darrow et al. 2022). Epidural SCS (ESCS) uses implanted electrodes, while transcutaneous SCS (tSCS) is a non-invasive alternative (Rademeyer et al. 2021; Shealy et al. 1967). Studies combining ESCS with body-weight supported treadmill training (BWSTT) or overground walking have shown improvements in walking speed, endurance, and voluntary leg movement in people with incomplete or motor-complete SCI.
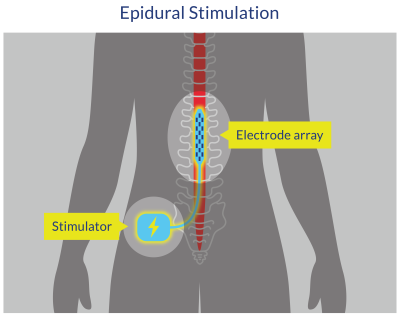
Figure 10. Epidural Spinal Cord Stimulation (ESCS)
Carhart et al. (2004) and Herman et al. (2002) were among the first to describe the effects of ESCS combined with gait training in one person with SCI (male with incomplete tetraplegia, 43 years old, injury level C5-C6, AIS C, 3.5 years post-injury). The participant first underwent 12 weeks of BWSTT, which resulted in some significant improvements in treadmill gait parameters, although his walking overground remained limited. Subsequently, the participant underwent surgical implantation of an epidural stimulation system placed over the T10-T12 vertebral level. After surgical healing had taken place, the participant resumed walking training with both BWSTT and OGT. The combination of ESCS with gait training resulted in a substantial improvement in treadmill gait parameters as well as in overground ambulation. The participant reported a decreased sense of effort, a doubling in walking speed, increased walking endurance, including walking outdoors in his community, when assisted by spinal cord stimulation.
More recently, Harkema et al. (2011) described the effect of ESCS in combination with LT in a single male participant with a motor complete spinal cord injury (23 years old, injury level C7-T1, AIS B, 3.4 years post-injury) (Harkema et al. 2011). Before implantation, the participant underwent 170 LT sessions and was unable to stand or walk independently or voluntarily move his legs. A 16-electrode array was surgically placed on the dura (L1-S1 cord segments). Optimal stimulation parameters for standing and stepping were tested. With stimulation, the participant was able to maintain standing unassisted with full weight-bearing. Locomotor-like muscle activity patterns emerged when epidural stimulation was combined with BWSTT (but not without stimulation). Interestingly, the participant was also able to regain some ability to voluntarily move the legs (but only in the presence of the epidural stimulation). Further studies have extended these initial findings to other people with motor-complete SCI (Angeli et al. 2018; Angeli et al. 2014; Calvert et al. 2019; Darrow et al. 2019; Gill et al. 2018; Gorgey et al. 2020b; Rejc et al. 2015; Rejc et al. 2017), and even allowing for recovery of voluntary leg movement and standing without the ESCS (Reck & Landmann 2017).
Similar to ESCS, the laparoscopic implantation of neuroprosthesis (LION) procedure in the pelvic lumbosacral nerves (i.e., sciatic, pudendal, and femoral nerves) has been developed. LION consists of the laparoscopic implantation of fine wire electrodes (for simulation of the spinal cord or sacral nerve roots) in direct contact with the endopelvic portion of the nerves for neuromodulation (Possover 2014). In 2006, the first laparoscopic implantation of a neuroprosthesis to the pelvic nerves was performed on a patient with paraplegia for the control of bladder function (Possover et al. 2010). Over the last 10 years, several studies have revealed that stimulation of pelvic nerves might indeed be capable of inducing neurologic changes for the recovery of leg movements in people with chronic SCI (Possover 2014; Possover & Forman 2017; Possover 2021).
Transcutaneous spinal (direct) current stimulation (tSCS) is a mild, noninvasive form of ES targeting modulation of spinal reflexes, corticospinal excitability, and spinal processing of sensory inputs (Hawkins et al. 2022). tSCS uses non-invasive electrodes placed over the T12—L1 vertebrae and abdomen, and has been shown to recruit similar neural structures as ESCS (Al’joboori et al. 2020). This simple method removes the need for surgery, and can be readily transferable for clinical use; however, it can cause some discomfort and unwanted muscle contractions as the current passes through skin and trunk musculature, has less specificity than ESCS, and may be more affected by changes in body position (Al’joboori et al. 2020; Danner et al. 2016). tSCS, delivered at an intensity that is sub-threshold for generating lower limb activity (muscle contractions or whole-limb responses), but high enough to produce paresthesia in lower limb dermatomes, has also been shown to augment volitional stepping on a treadmill (Hofstoetter et al. 2013; Hofstoetter et al. 2015) in people with incomplete SCI (Al’joboori et al. 2020).
