Transcranial Direct Current Stimulation (tDCS) for Standing Balance
Transcranial direct current stimulation (tDCS) is increasingly used in rehabilitation research as a neuromodulation approach to influence the excitability of cortical and cerebellar networks (Evans et al. 2022). The aim is often to “prime” neural circuits to increase corticospinal activation and to augment the effects of motor skill training (Evans et al. 2022). The majority of the studies published on the combination of tDCS and motor rehabilitation is related to upper limb, and studies examining the effects of tDCS on lower limb motor learning in persons with motor-incomplete SCI are still limited (Kumru et al. 2016).
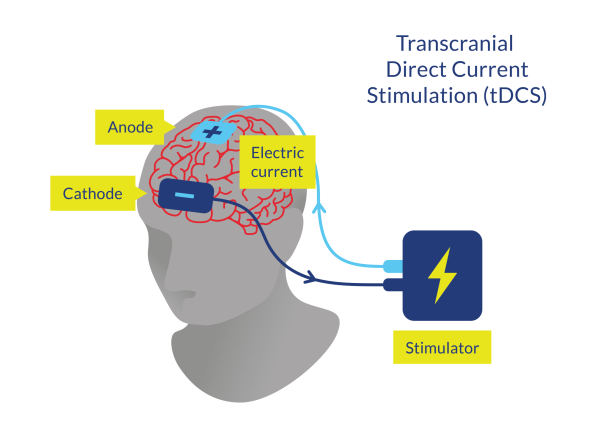
Figure 4. Transcranial Direct Current Stimulation (tDCS)
Discussion
One high-quality study assessed the effects of the combination of tDCS and motor skill training in patients with chronic motor-incomplete SCI (Evans et al. 2022). Patients received the intervention during three consecutive days and were randomly allocated to one of two groups: 20 min of tDCS delivered concurrently with motor skill training (patients had to perform a circuit of 6 motor task activities of standing balance exercises for 60 s, repeated 4 times) or a sham application of tDCS in combination with the same training intervention (Evans et al. 2022). It was shown that the locomotor-related motor skill training, with and without tDCS, provided significant increases in overground walking speed (10MWT), walking distance (2MWT), cadence, bilateral stride length, and balance function (BBS and FES-I), but the addition of tDCS was not associated with greater improvements compared with the sham application (Evans et al. 2022). Another RCT assessed participants with incomplete chronic SCI who were assigned to receive anodal tDCS over the vertex while sitting or to a sham tDCS application (Klamruen et al. 2024). After the tDCS application, all participants underwent overground gait training at a moderate intensity (Klamruen et al. 2024). After 5 days of consecutive sessions, both groups showed similar significant improvements in standing balance (TUG); however, higher improvements were observed in the experimental group for walking speed (10MWT) (Klamruen et al. 2024).
Two high-quality studies assessed the effects of the combination of tDCS and BWSTT in patients with chronic and motor-incomplete SCI (Simis et al. 2021; Raithatha et al. 2016). In the RCT of Simis et al. (2021), patients with chronic SCI received 20 min of active or sham tDCS immediately before BWSTT with Lokomat for 30 min. After 30 sessions, there was a statistically significant difference in the percentage of participants that improved in WISCI II (33% in the sham group vs. 70% in the active group) and in the follow-up; however, other outcome measures related to walking (10MWT and 6MWT) or balance (BBS and TUG) improved significantly in both groups, but without statistical differences between groups (Simis et al. 2021). Lastly, the study of Raithatha et al. (2016) was the very first to evaluate the effects of combining tDCS with locomotor training to facilitate gait recovery for people with incomplete and chronic SCI. Patients were randomly allocated into two groups: 20 min of tDCS immediately before locomotor training with Lokomat (Raithatha et al. 2016). Balance, as measured by BBS, improved in both groups nearly equally (and TUG improved only in the sham group), suggesting that while LT-RGO can improve balance, tDCS may not enhance this improvement (Raithatha et al. 2016).
It should be noted that tDCS seems to be a feasible and safe intervention. Studies by Raithatha et al. (2016) and Simis et al. (2021) stated that all patients tolerated the intervention with no complications. Evans et al. (2022) and Klamruen et al. (2024) found that four participants in the active tDCS group (n=14) had mild-to-moderate poststimulation headache related to the tDCS intervention (but it was not stated if the AE occurred in the active or the sham group), and 42-44% of participants in the experimental group reported itching and tingling sensations, respectively.
Conclusions
There is level 1 evidence (from 1 RCT: Evans et al. 2022) that a brief intensive motor skill training involving a circuit of ballistic, cyclic locomotor-related skill activities improved overground walking speed (10MWT), walking distance (2MWT), cadence, bilateral stride length, and balance function (BBS and FES-I); however, concurrent application of tDCS did not further enhance the effects of motor skill training in patients with motor-incomplete SCI.
There is level 1 evidence (from 1 RCT: Klamruen et al. 2024) that anodal or sham tDCS in a sitting position prior to overground gait training at moderate intensity for five days provide similar improvements in standing balance (TUG), but higher improvements in 10MWT for the anodal tDCS in patients with incomplete and chronic SCI.
There is level 1 evidence (from 2 RCTs: Simis et al. 2021; Raithatha et al. 2016) that the application of tDCS immediately before BWSTT with Lokomat improved significantly more the walking ability (WISCI II) and lower extremity motor function (manual muscle testing), and similarly the walking speed (10MWT), walking distance (6MWT) and balance (BBS and TUG), compared to the same intervention but with sham stimulation in patients with motor-incomplete and chronic SCI.
