Pathophysiology of Heterotopic Ossification
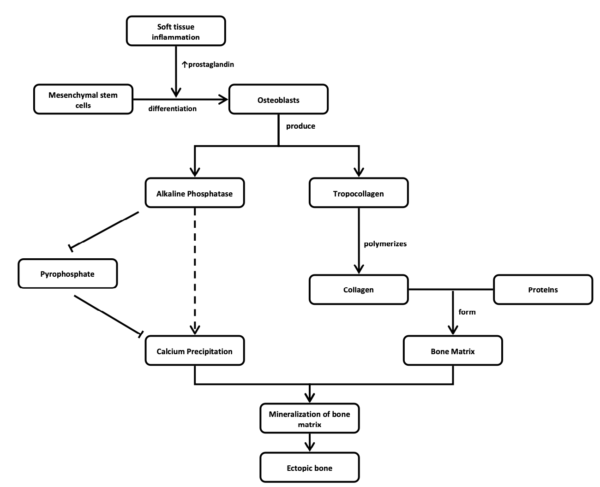
The mechanism underlying HO following spinal cord injury is not fully understood creating challenges in early diagnostic and therapeutic interventions. In the body, bone tissues are maintained by three primary cell types; osteoblasts, osteocytes, and osteoclasts (Findlay, 2018). HO appears to be initiated by metaplasia of mesenchymal cells into bone precursor cells (Schuetz et al. 2005). Mesenchymal stem cells can differentiate into osteogenic cells given the right stimuli and within the right environment (even soft tissue; Chalmers et al. 1975; Pape et al. 2004). These mesenchymal stem cells can generate cartilage, muscles, tendons, ligaments or fat, besides bone (Williams et al. 1999) and are thought to play a pivotal role in the development of HO (Pape et al. 2004). After mesenchymal cell differentiation to osteogenic cells, a protein mixture created by bone cells (osteoid) calcifies within a matter of weeks (Pape et al. 2001). Over the next few months, the calcified osteoid remodels and matures into well-organized trabecular bone (Pape et al. 2001). Months following the initial trauma, patients develop bone formation in muscle and soft tissues in an ectopic location with resultant restriction in range of motion, pain and ankyloses at the affected joint (Banovac & Gonzalez 1997; Garland et al. 1980). The bony lesion has a high metabolic rate, adding new bone at more than three times the rate of normal bone. Osteoclastic (bone removal cells) density is more than twice that found in healthy bone (Puzas et al. 1987). It is suspected there may be a neurogenic factor contributing to HO but the mechanism is poorly understood (Hurvitz et al. 1992; Pape et al. 2001; Pape et al. 2004).
Another recent theory for the development of HO has been suggested (Brady et al. 2018). As an inflammatory response is triggered by CNS damage, the inflammatory cells involved, such as neutrophils, lymphocytes, and macrophages, are thought to release a variety of growth factors and cytokines (Tannous et al. 2013). These inflammatory lesions are thought to eventually turn into bone through a hypoxic microenvironment ultimately leading to mesenchymal condensations at peripheral injury sites (Wang et al. 2016; Agarwal et al. 2016; Winkler et al. 2015).