Characteristics/ Predictors of OH
Orthostatic hypotension (OH) is a common cardiovascular complication following SCI, caused by impaired sympathetic control, loss of vasoconstriction reflexes, and excessive blood pooling in the lower body. Individuals with higher-level injuries, particularly tetraplegia, are at greater risk, and factors such as low plasma volume, cardiovascular deconditioning, and post-prandial hypotension can further contribute. OH is characterized by drops in blood pressure and compensatory increases in heart rate upon standing, with variable responses depending on injury level, completeness, and autonomic function. Understanding these predictors and hemodynamic patterns is essential for identifying and managing OH in people with SCI.
Two major causes of OH can be attributed to the low level of efferent sympathetic nervous activity and the loss of the vasoconstriction reflex following SCI. Additionally, decreases in BP following the change to an upright position in individuals with SCI may be related to excessive pooling of blood in the abdominal viscera and lower extremities (Krassioukov & Claydon 2006; Claydon et al. 2006; Mathias, 1995). This decrease in BP is compounded by the loss of lower extremity muscle function post-SCI that is known to be important in counteracting venous pooling in the upright position. Excessive venous pooling in the lower extremities coupled with reduced intrathoracic blood volume lowers ventricular end-diastolic filling pressure and volume, therby reducing left ventricular stroke volume (Ten Harkel et al. 1994). Ultimately, this decreases cardiac output and arterial pressure, triggering arterial baroreceptor unloading which induces a reflexive reduction in cardiac parasympathetic (vagal) activity. As a result, HR increases but is often insufficient to compensate for the decreased stroke volume. Moreover, continued pooling of blood in the lower extremities may further reduce cerebral flow, causing symptoms of cerebral hypoperfusion such as dizziness, blurred vision, light-headedness, and fainting.
Several other factors may predispose individuals with SCI to OH, including low plasma volume, hyponatremia, and cardiovascular deconditioning due to prolonged bed-rest (Claydon et al. 2006; Illman et al. 2000; Mathias, 1995). The prevalence of OH is greater in individuals with higher spinal cord lesions, and thus it is more common in people with tetraplegia (Claydon et al. 2006; Mathias, 2006; Frisbie & Steele 1997). Furthermore, individuals with cervical SCI also experience greater posture-related decreases in blood pressure than those with paraplegia (Claydon et al. 2006; Mathias, 1995). There is also an increased risk of OH in individuals who sustain a traumatic SCI versus a non-traumatic injury such as spinal stenosis (McKinley et al. 1999).
In addition to central causes of OH following SCI, there is also evidence that suggests peripheral mechanisms could contribute to orthostatic intolerance following SCI. For example, up-regulation of the potent vasodilator, nitric oxide (NO), could potentially contribute to the orthostatic intolerance in people with SCI (Vaziri, 2003). In animal studies, it has been shown that NO synthase expression is dysregulated following SCI (Zhao et al. 2007). Moreover, Wecht et al. (2007) found that intravenous infusion of a relatively low dose of the NO synthase inhibitor, L-arginine-N-methyl-ester (L-NAME), normalized blood pressure in individuals with SCI.
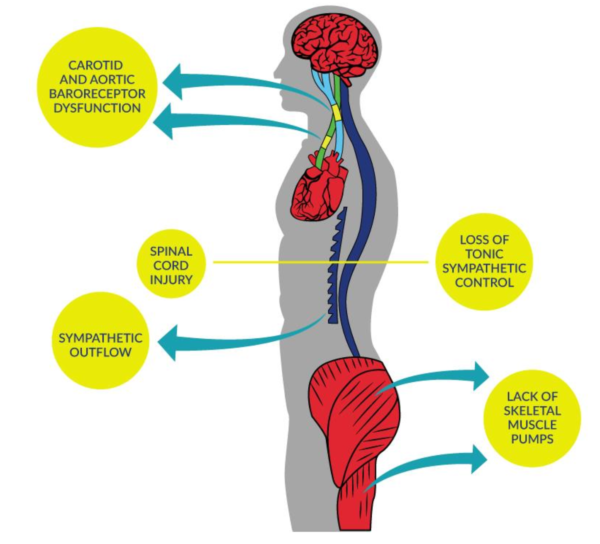
Figure 2. Blood Pressure Dysfunction due to SCI
Table 2. Factors Contributing to OH Following SCI
| Loss of tonic sympathetic control | Houtman et al. 2000; Wallin & Stjernberg 1984 |
| Altered baroreceptor sensitivity | Wecht et al. 2003; Munakata et al. 1997 |
| Lack of skeletal muscle pumps | Faghri & Yount 2002; Raymond et al. 2002; Ten Harkel et al. 1994 |
| Cardiovascular deconditioning | Hopman et al. 2002; Vaziri, 2003 |
| Altered salt and water balance | Frisbie, 2004 |
| Multifactorial | Claydon et al. 2006 |
Discussion
Literature in SCI and OH is consistent in their findings, but there is some variability across studies. Consistently, studies (Park et al. 2024; Stampas et al. 2019) suggest decreases in sBP and dBP accompanied by increases in HR upon assumption of an upright position from supine, often meeting the criteria for OH. However, Vaccaro et al. (2022) did not find a change in sBP upon sitting in individuals with SCI.
Wang et al. (2020) found that some people without OH criteria still experienced drops in sBP of 10-20 mmHg, drops in dBP of 5-10 mmHg, and increase in HR of greater than 10 BPM. Wang et al.’s (2020) cluster analysis of hemodynamic responses to a seated position identified eight patterns of interaction between BP and HR during orthostatic stress, indicating varied autonomic responses (e.g., Cluster 1: large decreases in sBP and dBP; moderate rise in HR; Cluster 3: large decreases in sBP and dBP; large rise in HR). This suggests that BP instability cannot be wholly predicted by level and completeness of SCI, and the consensus statement definition of OH is insufficient to characterize the variability of BP and HR responses during orthostatic stress (Wang et al. 2020).
In people without SCI, sBP, dBP, and HR decreases while asleep (nocturnal dip) and increases when awake, while individuals with SCI and OH will not show a nocturnal dip (Wang et al. 2022). However, participants with SCI in a study by Wang et al. (2022) demonstrated a reversed nocturnal dip, and sBP, dBP, and HR decreased while awake compared to asleep.
Post-prandial hypotension is another symptom commonly experienced by people with SCI. Post-prandial hypotension is defined as a decrease of ≥20 mmHg in SBP or a SBP of <90 mmHg after having been >100 mmHg before the meal within two hours after a meal (Hansen et al. 2021). A study by Hansen et al. (2021) found that 49.4% of their participants met the criteria for post-prandial hypotension, and in 4.4% of participants, the drop in sBP was accompanied by symptoms of hypotension. Moreover, 17% of post-prandial hypotension episodes occurred simultaneously with transfers, while 23% of episodes occurred with physical activity (physical exercise, physiotherapy, and occupational therapy (Hansen et al. 2021). Level of completeness of SCI also affects an individual’s likelihood of getting PPH; one study shows people with complete injuries have a 9x higher risk of PPH than people with incomplete injuries (odds ratio: 9.482, p=0.000) (Hansen et al. 2021).
Conclusion
There is level 2 evidence (from one prospective controlled trial) (Park et al. 2024) that mean HR is lower in individuals with symptomatic OH compared to asymptomatic OH.
There is level 2 evidence (from one prospective controlled trial) (Stampas et al. 2019) that individuals with SCI and OH have lower BP and higher HR compared to individuals with SCI without OH and controls.
There is level 2 evidence (from one prospective controlled trial) (Wang et al. 2022) that individuals with SCI do not show a nocturnal dip in sBP, dBP, and HR like individuals without SCI, but rather show an increase in sBP, dBP, and HR during sleep (reversed nocturnal dip).
There is level 2 evidence (from one prospective controlled trial) (Wang et al. 2020) that individuals with SCI present drops in sBP and dBP accompanied by increases in HR upon the assumption of an upright position.
There is level 4 evidence (from one cohort study) (Hansen et al. 2021) that post-prandial hypotension was experienced by 49.4% of participants.
There is level 4 evidence (from one case series) (Kee et al. 2021) that presyncopal symptoms occur when the CBFV decrease is more than 21% after a 50 degree tilt.
There is level 4 evidence (from one pre-post) (Vaccaro et al. 2022) that a sit-up test did not induce changes in sBP in individuals with SCI.
There is level 5 evidence (from one observational study) (Katzelnick et al. 2019) that average sBP, dBP, and HR is significantly lower in individuals with tetraplegia compared to individuals with low thoracic SCI.
